Prostate cancer is the most frequently diagnosed cancer and the second leading cause of cancer-related deaths among men in the United States. If detected at early stages the prognosis is quite favorable; however, aggressive forms of metastatic prostate cancer spread primarily to the skeleton. Bone tumors cause great pain, promote fractures, and ultimately represent the main cause of morbidity, with a 70% incidence documented by autopsies. Dynamic interaction between prostate cancer and the bone microenvironment is a major contributor to prostate cancer metastasis to bone. It has been hypothesized that the bone microenvironment serves as a rich “soil” by secreting factors that promote survival and propagation of cancer cells; in turn tumors secrete factors that alter the bone microenvironment to promote metastatic colonization. Development of new therapies for the prevention and treatment of prostate cancer bone metastasis depends on understanding the dynamic reciprocal interactions between prostate cancer and the bone microenvironment.
A collaboration involving scientists from Lawrence Livermore National Lab and the University of California campuses at Merced and Davis has found that Sclerostin (SOST), a secreted protein predominantly expressed in bone, inhibits prostate cancer metastasis to bone through reciprocal molecular changes in both cancer and bone cells. Two accompanying studies conducted in Dr. Gabriela Loots’ laboratory, a biomedical scientist at LLNL and an associate adjunct professor at the University of California, Merced appear online in the journal PLOS ONE [journals.plos.org/plosone/article?id=10.1371/journal.pone.0142058] and Microarrays [http://www.mdpi.com/2076-3905/4/4/503].
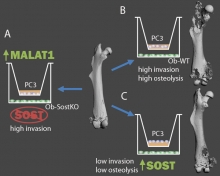 The first study led by Aimy Sebastian, a graduate student in the School of Natural Sciences, at UC Merced, who is pursuing her doctorate degree under Dr. Loots’ mentorship identified SOST as a key molecule dysregulated as a result of prostate cancer-bone microenvironment interactions. This study, published in the journal Microarrays, shows that lack of SOST in the bone microenvironment promotes the expression of many genes associated with cell migration and/or invasion including long non-coding RNA MALAT1 in prostate cancer suggesting that SOST has an inhibitory effect on prostate cancer invasion. The second study led by Bryan Hudson, a postdoctoral fellow and Nicholas Hum, a biomedical scientist in the Physical and Life Sciences Directorate at LLNL, investigated the role of SOST in regulating prostate cancer invasion and metastasis, in vitro and in animals. This study showed that SOST inhibits prostate cancer invasion in vitro. To determine whether SOST impacts metastasis, they modified prostate cancer cell line, PC3, to overexpress SOST, engrafted the various PC3 cells lines onto immunodeficient mice, and quantified the rate of secondary tumors and of osteolytic bone lesions. They found that PC3 cells producing more SOST had significantly lower rates of metastasis. In addition, with the help of Dr. Blaine Christiansen, an assistant professor in the Department of Orthopedic Surgery, at UC Davis, they found that PC3 cells expressing more SOST induced significantly less osteolytic bone loss than PC3. These results provided strong evidence that SOST has an inhibitory effect on prostate cancer metastasis to bone.
The first study led by Aimy Sebastian, a graduate student in the School of Natural Sciences, at UC Merced, who is pursuing her doctorate degree under Dr. Loots’ mentorship identified SOST as a key molecule dysregulated as a result of prostate cancer-bone microenvironment interactions. This study, published in the journal Microarrays, shows that lack of SOST in the bone microenvironment promotes the expression of many genes associated with cell migration and/or invasion including long non-coding RNA MALAT1 in prostate cancer suggesting that SOST has an inhibitory effect on prostate cancer invasion. The second study led by Bryan Hudson, a postdoctoral fellow and Nicholas Hum, a biomedical scientist in the Physical and Life Sciences Directorate at LLNL, investigated the role of SOST in regulating prostate cancer invasion and metastasis, in vitro and in animals. This study showed that SOST inhibits prostate cancer invasion in vitro. To determine whether SOST impacts metastasis, they modified prostate cancer cell line, PC3, to overexpress SOST, engrafted the various PC3 cells lines onto immunodeficient mice, and quantified the rate of secondary tumors and of osteolytic bone lesions. They found that PC3 cells producing more SOST had significantly lower rates of metastasis. In addition, with the help of Dr. Blaine Christiansen, an assistant professor in the Department of Orthopedic Surgery, at UC Davis, they found that PC3 cells expressing more SOST induced significantly less osteolytic bone loss than PC3. These results provided strong evidence that SOST has an inhibitory effect on prostate cancer metastasis to bone.
Additional contributors working with Dr. Loots on these studies were Cynthia Thomas, Ayano Kohlgruber, Nicole Collette and Matthew Coleman from Lawrence Livermore National Lab.
This work was supported by LLNL LDRD-10-ERD-020 and Developmental Grant from UC Davis Cancer Center and was conducted under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Caption: Modulating Sost expression in osteoblasts affects prostate cancer invation and metastasis. PC3 cells co-cultured with osteoblasts lacking Sost (Ob-SostKO) are more invasive and up-regulate non-coding RNA MALAT1 (Sebastian et al. 2015) (A). PC3 cells co-cultured with wildtype osteoblasts (Ob-WT) are invasive and form aggressive osteolytic lesions (B) while overexpression of Sost reduces invasion and inhibits osteolytic lesions (Hudson et al. 2015) (C).

